Electrochemistry is the study of chemical processes involving electricity. In some chemical reactions, the generated electrical current can be used to do useful work, but in others, it can be used to speed up the reaction. Many useful battery technologies and everyday household things are based on these reactions.
Electrochemistry refers to the study of chemical reactions that can cause electrons to move around in their orbits. A chemical reaction that involves both oxidation and reduction, also referred to as a “redox” reaction, is a process that generates electricity by moving electrons from one element to another.
What is redox reaction?
A chemical process known as an oxidation-reduction reaction occurs when two distinct species exchange electrons with one another (redox). An oxidation-reduction reaction can be defined as any chemical reaction in which an electron can be gained (via reduction) or lost (through oxidation) by a molecule, an atom, or an ion.
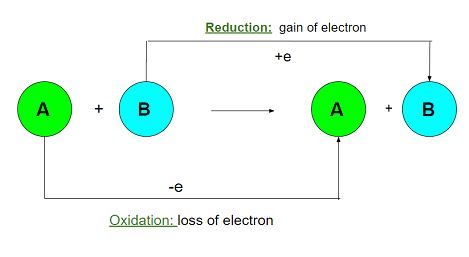

Electrochemistry studies the overall reactions that occur when many redox processes are coupled by an external electric current and a suitable electrolyte. Electrochemistry involves chemical reactions that separate charges. Homogeneous or heterogeneous charge transfer is common in charge dissociation.
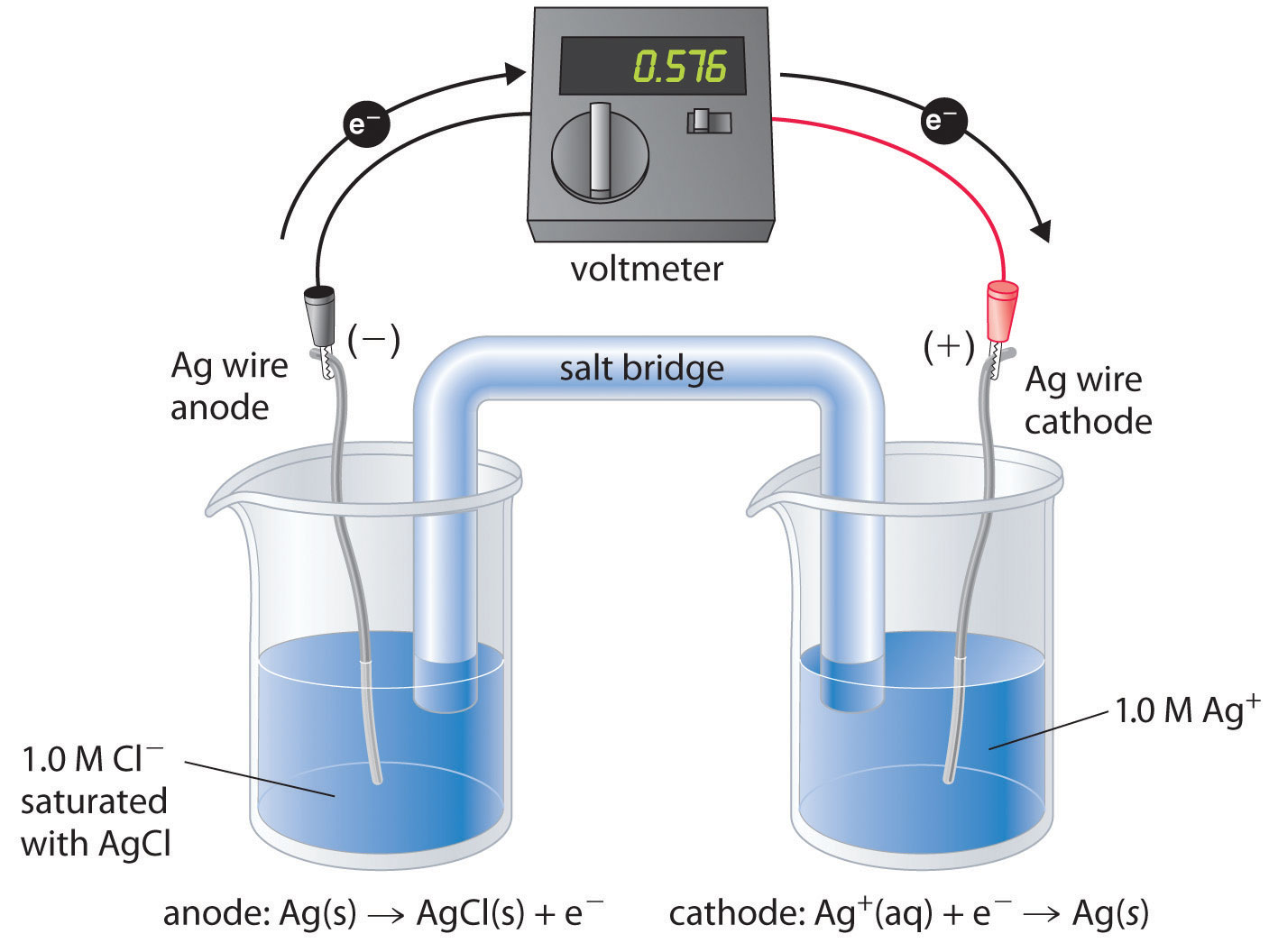
Electrochemical Cells
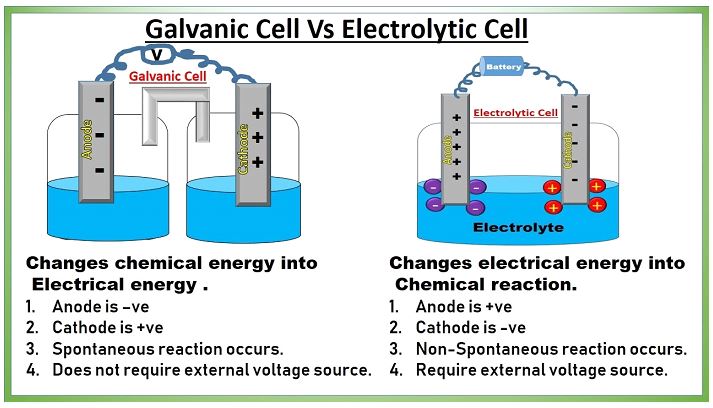
A spontaneous reaction is a chemical reaction that occurs without the intervention of the observer. Chemical energy is converted into electric power when a spontaneous reaction (also known as a redox reaction) occurs in electrochemistry. The process can be reversed by adding electricity to a non-spontaneous chemical reaction. To make these changes, electrochemical cells are utilized. There are two types of electrochemical cells in electrochemistry which are explained below.
Galvanic Cell
Through a process known as a redox reaction, the galvanic cell is able to produce electricity by transforming the chemical energy that is present into electrical energy. Oxidation and reduction take place in their own distinct areas of the device. Each compartment contains an electrolyte solution as well as a metallic conductor that acts as an electrode. A half-cell is the name given to the compartment that houses the electrode in addition to the electrolyte solution.
Electrolytic Cell
Within the electrolytic cell, charge is moved from the electrical realm to the chemical one so that reactions can take place. At this stage of the process, the electrodes are submerged in an electrolytic solution that is composed of both cations and anions. When an electric current is run through the system, the ions will move to the electrodes with the opposite polarity, where they will simultaneously go through the processes of reduction and oxidation. This will occur when the ions encounter an electric field.

Check out other cool chemistry concepts with other chemistry kits below!