In compounds, chemical bonds hold atoms of different elements together. Chemical bonding permits an endless number of new compounds and elemental combinations to be created.
How is a Chemical Bond Formed?
A chemical bond is formed when the outside (valence) electrons of one atom are attracted to the positive nuclear charge of its neighbor. Some materials require activation energy to overcome their initial repulsive forces. When atoms are separated by the ideal distance, which can be calculated by altering the distance between them, electrostatic forces are lowered. The bond length is the distance between the atoms at this moment, and this minimum represents the most stable state.
Types of Chemical Bonds
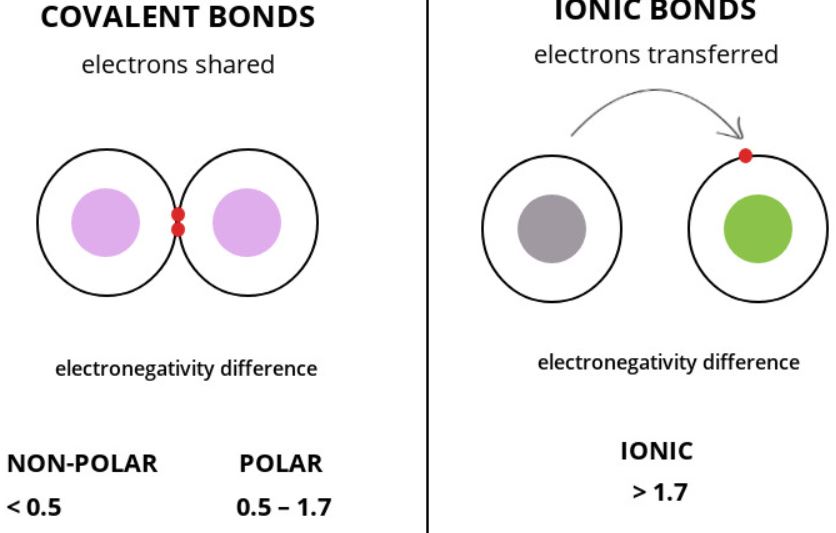
There are at least a dozen different types of chemical interactions and linkages. The two most common types of chemical bonds, covalent and ionic bonding, will be discussed in this article.
Ionic Bond
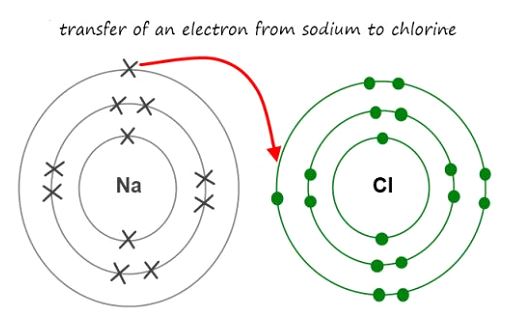
Electrovalent bonds, also known as ionic bonds, are created when two ions with opposite charges in a molecule attract one other. To create this kind of connection, all of one atom’s outermost valence electrons must be transferred to the other.

Properties of Ionic Bonds
⦁ Ionic bonds are strongest of all types of chemical bonds.
⦁ Ionic bonds have high melting and boiling points.
⦁ Ionic bonds are non-volatile in nature.
⦁ Ionic bonds act as good insulators.
⦁ Ionic bonds are good conductors of electricity.
Electrons with opposing charges (one metal and one non-metal) are attracted to each other in the form of “ionic bonds”.
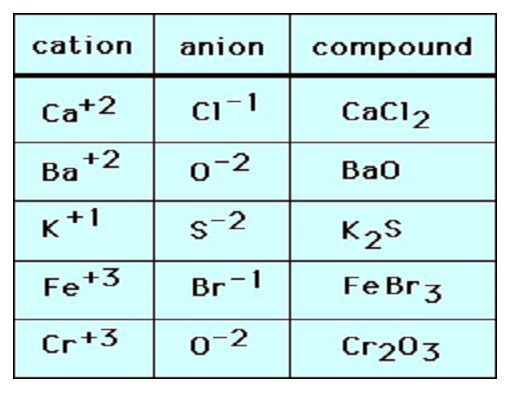

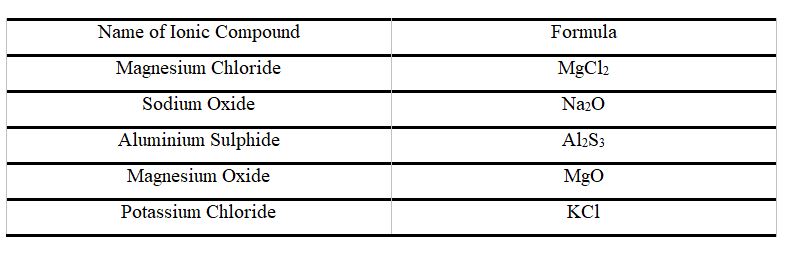
Some Other Examples of Ionic Bonds

Covalent Bonding
When two atoms trade one or more pairs of electrons with one another, this results in the formation of a covalent bond. These electrons are being subjected to competing pressures from the two nuclei of this atom. Ions can only be created when electrons are moved from one atom to another, but the difference in electronegativity between the two atoms is too small for this to be possible.
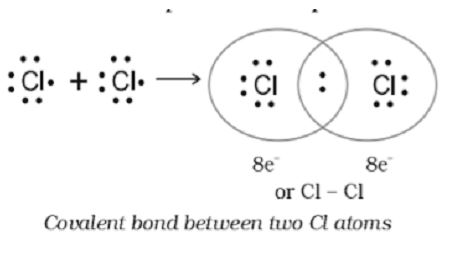

Properties of Covalent Bonds
⦁ Covalent bonds are not that hard, they are soft and flexible.
⦁ Covalent bonds have low melting and boiling points.
⦁ Covalent bonds do not conduct electricity.
⦁ Covalent bonds are insoluble in water.
⦁ Covalent bonds are soluble in organic solvents.
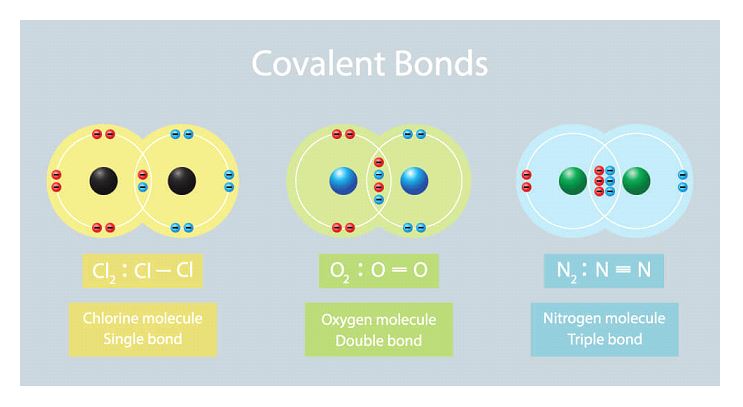

Some other Examples of Covalent Bonds
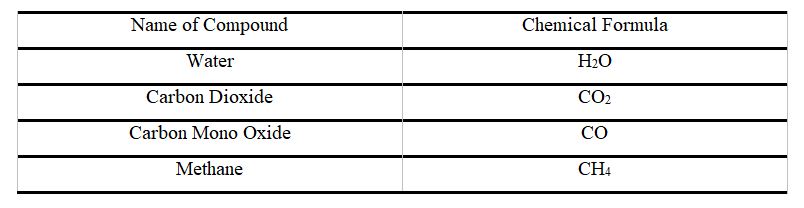

Check out other cool chemistry concepts with other chemistry kits below!