The branch of chemistry known as “nuclear chemistry” studies how atom nuclei change. These atomic alterations are what cause radioactivity and nuclear power to be produced in nuclear reactors.
The study of atomic nuclei and their ability to make new atoms is known as nuclear chemistry. During nuclear processes, the atoms themselves change. Nuclear reactions, like ordinary chemical reactions, are followed by energy shifts.
Types of Nuclear reactions
A number of nuclear reactions are studied under the branch of nuclear chemistry which are illustrated in the flow chart below:
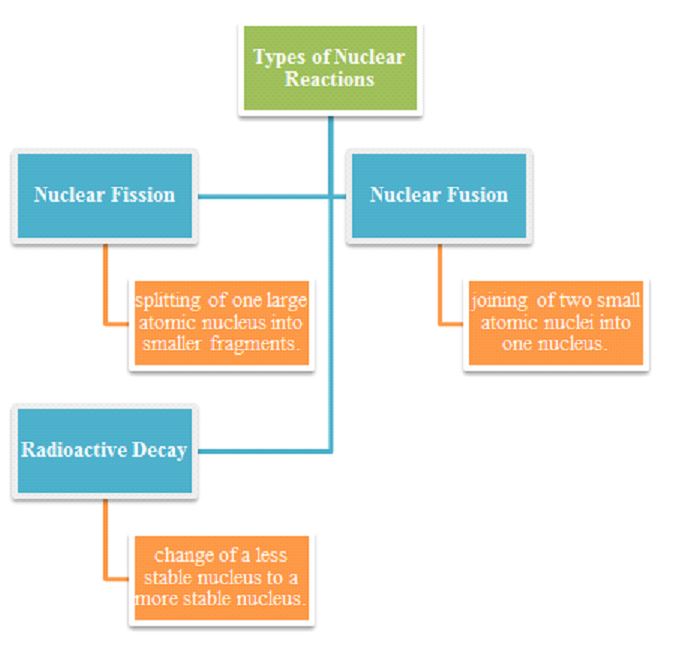

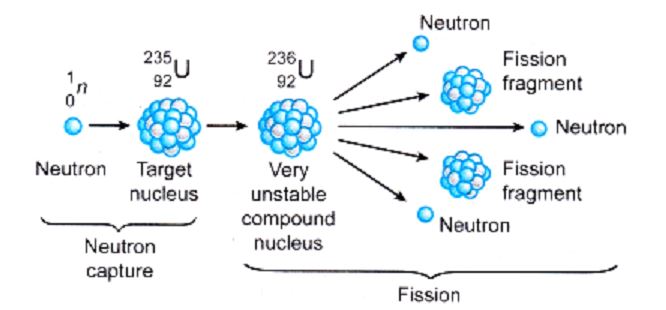
⦁ Nuclear Fission
The breaking of an atomic nucleus into two or more lighter nuclei is known as nuclear fission. This reaction could be the result of a nuclear reaction or radioactive decay. In nuclear fission reactions, neutrons and gamma rays are frequently emitted, resulting in massive amounts of energy (photons holding huge amounts of energy, enough to knock electrons out of atoms).

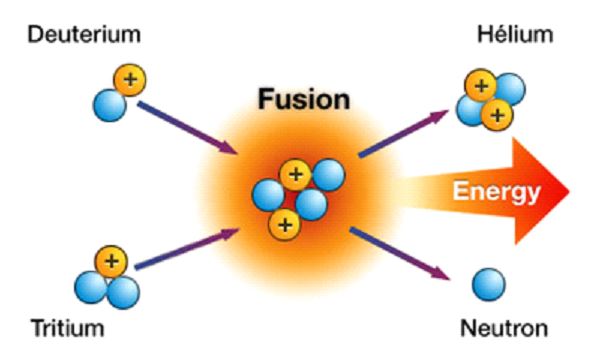
⦁ Nuclear Fusion
When two or more light nuclei combine, a heavier nucleus is formed. Hydrogen and its isotopes are fusion-capable elements with low atomic number. In the nuclear fusion reaction, which is the inverse of nuclear fission, heavy elements spread and generate lighter elements.

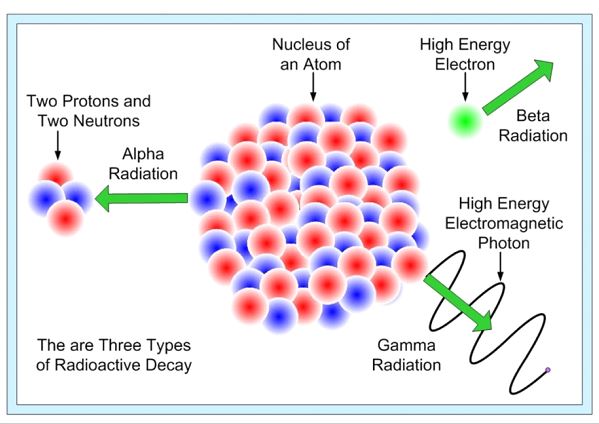
⦁ Radioactive Decay
When a nucleus undergoes radioactive decay, it loses energy, which results in the emission of radiation. These include gamma radiation, alpha particles (which are helium nuclei), and beta particles (which might be either electrons or positrons; high energy photons). As the energy of the nucleus drops, the nucleus’s ability to remain stable decreases as well.

A Few Applications of Nuclear Chemistry
Nuclear chemistry has a wide range of applications in our daily lives. Medicine and energy generating are the most well-known applications, but there are countless others, including agriculture and manufacturing.
Even as research expands the technology’s potential applications and justifications for widespread adoption, it will continue to play an increasingly vital role in our daily lives.
⦁ Medicine
Oncology, cardiology, neurology, pneumology, and pediatrics all use nuclear chemistry.
Diagnostic procedures such as X-ray imaging and radiation from radioactive materials or radiation-producing machines such as accelerators, as well as radiotherapy therapies that utilize the same techniques are used by medical professionals.
Among other things, nuclear technology can be used to sterilize medical equipment, study biological processes using tracers, and examine the qualities of tumorous cells.
⦁ Industry
The use of radioactive isotopes and radiation in industrial operations such as measurement, automation, and quality control is extremely beneficial. Currently, they are used in practically every field of study.
Trackers, for example, are used to collect data that extends the life of expensive industrial equipment. It is also possible to obtain X-rays of a component’s internal structure in order to assess its quality without affecting or changing the material’s composition.
⦁ Agriculture
Without relying on random mutations, scientists can employ radio isotopes and radiation techniques to modify plants and seeds to create specific agricultural types. Insect control, better crop yield, and reduced fertilizer consumption are all advantages of nuclear chemistry.
Check out other cool chemistry concepts with other chemistry kits below!