Let us learns about matter and the different states or phases in which it exists!
What is Matter?
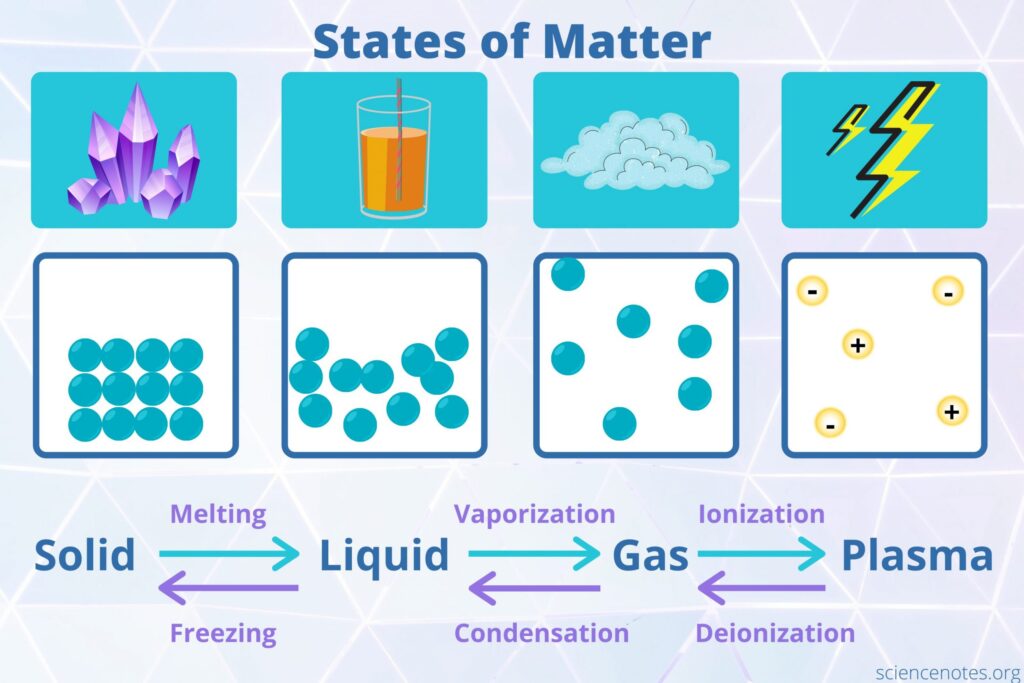
Matter is literally anything in the universe that occupies space and has mass. In our daily lives, we come across many living and non-living things that are described as matter. For example, the water you drink, the air you breathe, and even the people around you are matter. In short, our planet and the universe are composed of matter! Chemistry is the science that studies everything that exists in the universe. “Matter” is a more precise term for “everything”. Therefore, chemistry is the science of matter. Our planet earth, like the rest of our solar system’s planets, is made up of matter. All objects with mass that occupy space, including man-made objects and atmospheric gases, are classified as matter. A matter can exist in a number of different states. The four known states of matter are solid, liquid, gas, and plasma.
What are the Four States of Matter?

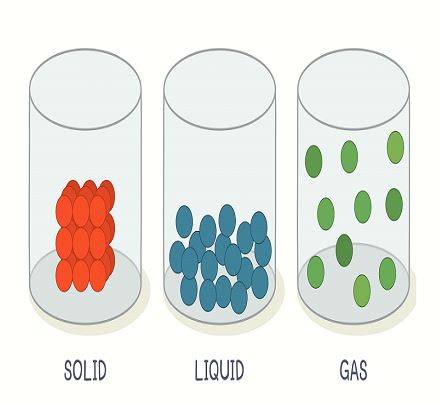
⦁ Solid state of matter
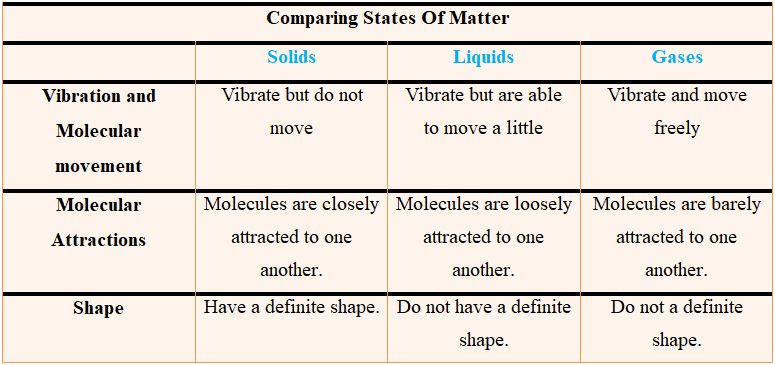
Solids are strong and tough. Solids have definite weight and volume. Molecular clusters that make solids are impermeable to movement because they are tightly packed. The human body is the most evident example of solid matter. A matter is said to be in a solid-state if it has a definite shape and mass. It is very difficult to compress the solids as the atoms and molecules making up the solids are closely and tightly packed together with minimum kinetic energy, fixing them at their specific position. This property enables solids to have a high density. Some of the common examples of solid state, you can look around are a computer, book, cup, car, etc.
⦁ Liquid State of Matter
It is possible to confine liquid state of matter in size or shape of container. Thickness and color are the only differentiating properties of liquid matter. Oceans, seas, and rivers are all excellent examples. Liquid molecules are lightly bound, enabling them to move about freely. The liquid state of matter has no definite shape but has a definite volume. Unlike solid, atoms in liquid are loosely packed and have the ability to move and flow around thus changing their shape. Liquids usually take the shape of the container containing them and have a low density as compared to solids. Like solids, they are also difficult to compress. Water, juices, soft drinks, milk, petrol, and oil, are all examples of liquids.
⦁ Gaseous State of Matter
This state of matter is present everywhere. We walk through gases without even noticing them. Molecules of gasses flow effortlessly because they are barely attached to one another. Oxygen (O2) is the air we breathe 24 hours a day but we can neither see it nor touch it. Another example of gaseous state of matter is helium gas which is present in balloons. As Helium gas is lighter than oxygen the balloons filled with helium float in air. The atoms and molecules in the gas state are far apart from each other, creating large spaces and moving freely. This results in no definite shape or volume of a gas. To avoid escaping, gas is usually filled in a container and can be easily compressed. The air you breathe is composed of many gases including oxygen, carbon dioxide, nitrogen, etc.
⦁ Plasma State of matter
In our everyday lives, this state of matter is naturally rare. These are highly charged gases in which the electrons have been removed. In nature, plasma may be found in the form of lightning and stars. It can be produced artificially in a number of ways by scientists. It is used in the manufacture of television screens for example. Plasma is a rare state of matter on Earth but common in the universe. It can generally be defined as a charged gas. The electrons in the plasma are not attached to atoms but are present in an excited state with very high kinetic energy giving plasma property of electrical conductance. The particles in the plasma move around freely and randomly. Flames and lightning are natural examples containing plasma. Neon signs and fluorescent lights also contain plasma. All the stars in the Universe are composed of super-heated plasma.
The three distinct states of matter most commonly found on earth are shown below in simplified form.


The image of the iceberg does not show plasma, but it does show the other three types of matter. Both the iceberg and the lake are made out of water, one being solid and other being liquid, respectively. Although it is invisible, gaseous water vapors are present in the atmosphere as well. Here is a concluding comparison of these 3 main states of matter found on earth.

Check out other cool chemistry concepts with other chemistry kits below!