Temperature
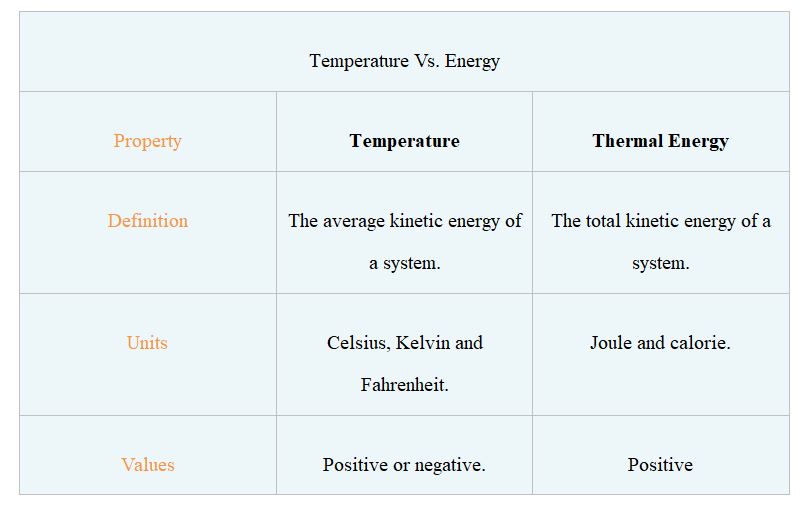
The average kinetic energy of particles in thermodynamic equilibrium in a macroscopic system is represented by temperature. This property of matter can be used to gauge heat emitted from it.
Temperature is important in the fields of physics, geology, chemistry, atmospheric science, and biology. A substance’s solubility, vapor pressure, and electrical conductivity, among other physical properties, are all affected by its temperature. Temperature has a significant impact on the rate and magnitude of chemical reactions.
Thermometers are devices that measure the temperature precisely. In academics and industry, three different temperature scales are now in use. The Celsius and Kelvin temperature scales are both included in the International System of Units. In the United States, Fahrenheit is the most regularly used temperature scale.
Energy
A physical property that describes a system’s ability to influence its surroundings or perform work is known as energy. Particles, entities, and systems can all be implicated in this phenomenon since it is so vast. There are various types of energy, each of which has its own name.
The total kinetic energy of a system’s structural components is known as thermal energy (atoms, molecules, and charged particles). This form of energy is linked to the mobility of the system’s structural components.
Relationship between Temperature and Energy
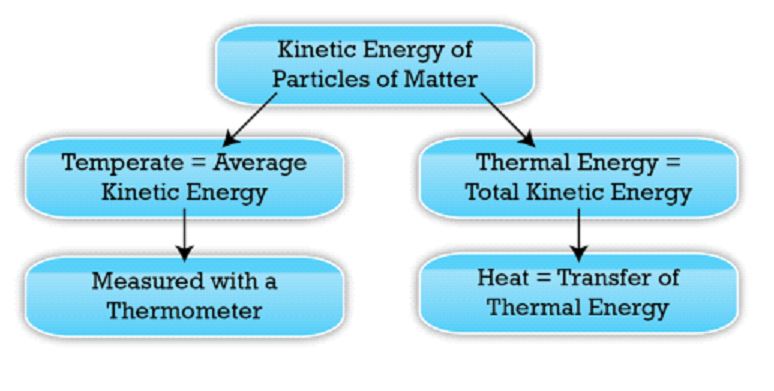

As the temperature rises, the kinetic energy of a body’s structural components increases. The thermal energy of an organism increases in direct proportion to its kinetic energy. As a result, as a person’s temperature rises, so does their thermal energy.
The amount of energy produced is proportional to the amount of weight carried by the substance. For example, to compare the densities of a cup of water and a lake at the same temperature, use the volume of each. At the same water temperature, molecules have the same average kinetic energy. In contrast, the quantity of molecules and thermal energy of water in a lake are significantly higher.
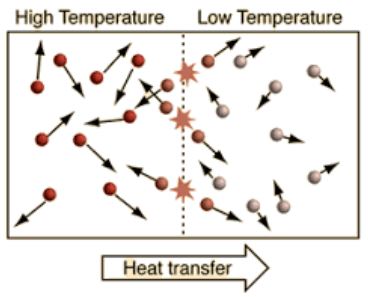
Thermal energy is exchanged when a temperature difference exists in a continuous matter system. Conduction, convection, and radiation are all methods for transferring heat energy. The heat is transferred from the hotter portions to the cooler ones. The practice is repeated until the temperature of the body (or system) is equalized.

Check out other cool chemistry concepts with other chemistry kits below!