Kinetics as the name implies is linked with motion or the bodies in motion affected by forces. In chemistry, kinetics is associated with the rates and speed of chemical reactions and is named chemical kinetics. Let’s learn about chemical kinetics!
What is Chemical Kinetics?
Chemical kinetics is the branch of physical chemistry that deals with the rates of chemical reactions. It determines how fast a reactant is converted into a product. It is a very important topic to learn as thermodynamics only deals with the direction of a reaction and does not tell us how fast it occurs. This information is provided by chemical kinetics.
Rate of Chemical Reaction
The rate of a chemical reaction is described in terms of the rate at which the reactants are consumed and the products are formed. It is important to note that as the reaction takes place, the concentration of reactants decreases, and that of the products increases. Generally, the concentration of substances in a chemical system is described as the quantity of a substance per unit volume. Therefore, the rate of a chemical reaction can be defined more conveniently as the concentration of reactants consumed and the concentration of product formed per unit of time. This can also be depicted by a graph between the concentration of products and reactants and the time of reaction as follows:
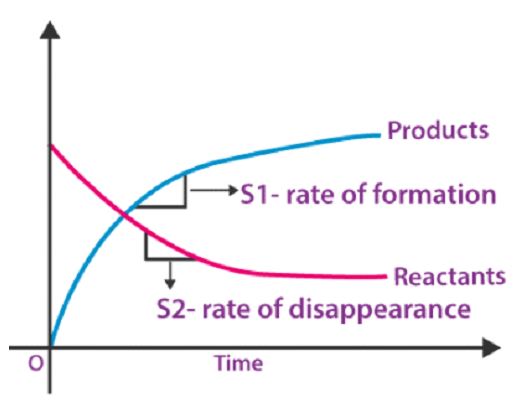

In the above graph, it is shown that at the time (t=0) the concentration of products is zero while reactants are present at a definite concentration. As the reaction proceeds with time, the concentration of reactants is gradually decreased and that of products is increased. It also shows that the slope of reactants is negative and that of products is positive.
Factors Affecting the Rate of Reaction
The following factors affect the reaction rates:
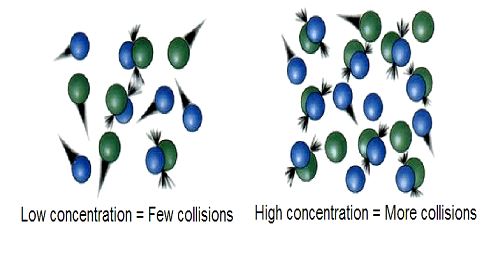
⦁ Concentration of reactants: As the reactants concentration increases, the number of collisions between the reactant molecules also increases (according to collision theory), which results in an increased rate of reaction.

⦁ Nature of reactants: The reactions involving atoms and ionic reactants proceed faster while those involving substances with covalent bonds are slower because time is required to break the bonds.
⦁ Physical state of reactants: Reactants in the same phase (solid, liquid, or gas) will come together more often without hindrance which increases the rate of reaction.
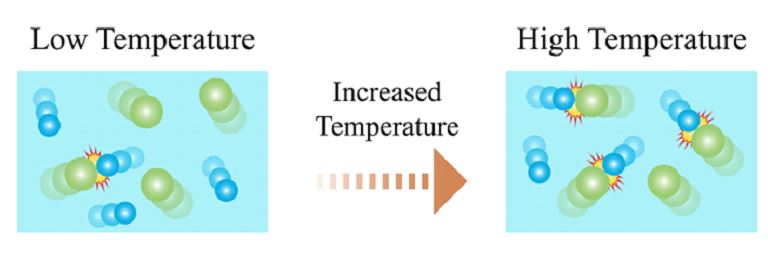
⦁ Temperature: Temperature is a major factor affecting the rate of reaction. As the temperature of a reaction system is increased, the reactant molecules will collide more frequently due to increased kinetic energy, which increases the frequency of collisions and hence the rate of reaction as shown below:

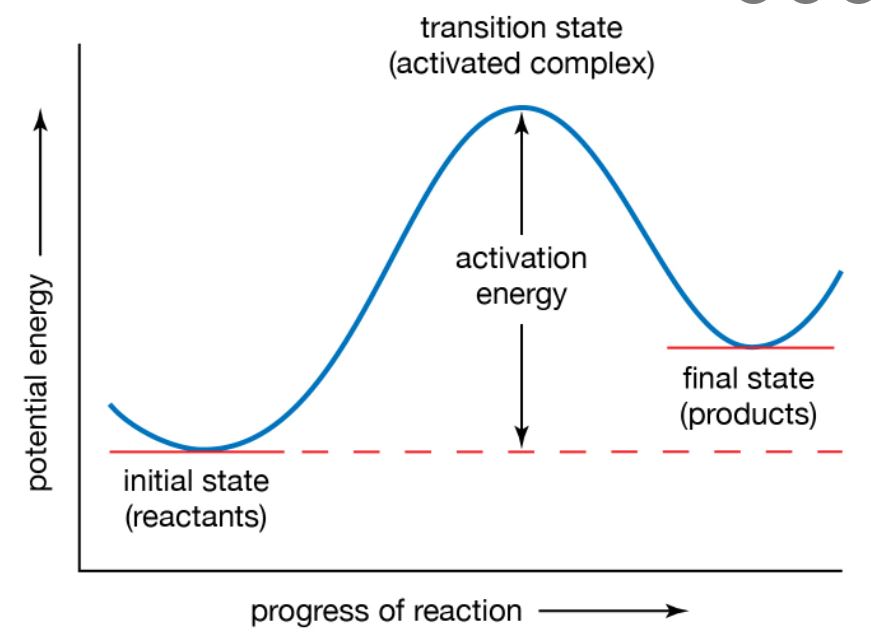
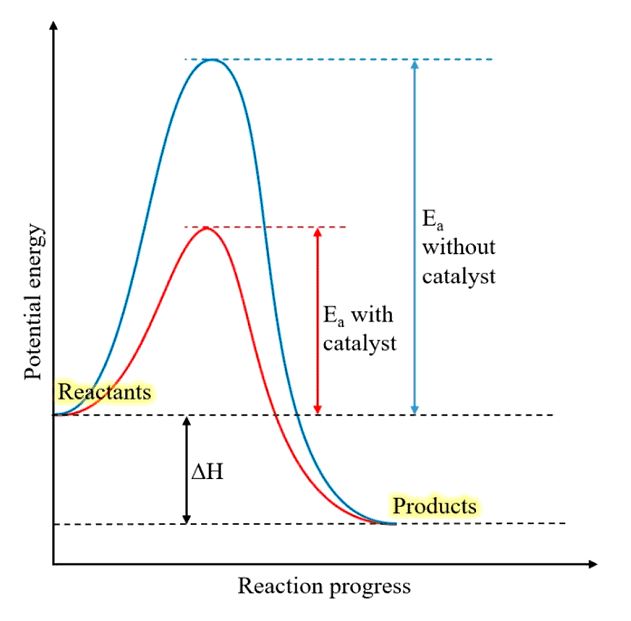
⦁ Catalyst: Catalysts also affect the rate of reaction by changing the mechanism of the reaction. Catalyst lowers the activation energy and results in the conversion of reactants into products more quickly as shown in the graph below.

Check out other cool chemistry concepts with other chemistry kits below!