What is Thermochemistry? Thermochemistry is the branch of thermodynamics that deals with the study of energy changes as the chemical reaction take place. The energy change is usually in the form of heat and it may be absorbed or released as a particular chemical reaction takes place. Therefore, thermochemistry also involves the exchange of energy between the system and its surroundings. Matter, energy, work, temperature, and entropy are the concerns of the four laws of thermochemistry. These four laws generally are observed with principles of enthalpy, Hess’s law, calorimetry, exothermic, and endothermic reactions listed below.
Enthalpy
The amount of heat/energy absorbed or released during a chemical reaction is called enthalpy of reaction represented by H and is usually measured as a change in enthalpy (ΔH) under constant pressure. It is represented as follows:
ΔH = qp
Where q represents the heat absorbed or released under constant pressure (p)
For a reaction, the change in enthalpy is also represented as the difference in the enthalpy of reactants and products:
ΔH = Hproducts – Hreactants
Enthalpy is measured in the units Kilojoule (kJ)
Based on this concept, chemical reactions can be divided into the following two types:
Exothermic reactions
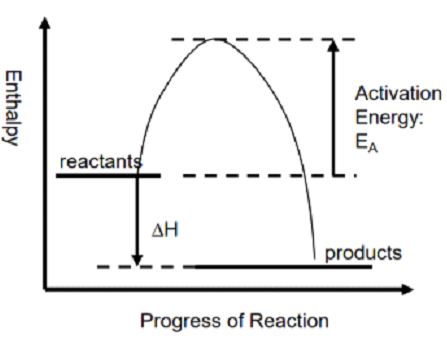
The chemical reaction involving the release of heat is called an exothermic reaction. In this case, energy is released in the surroundings and the temperature is increased. Products have less enthalpy as compared to the reactants, hence ΔH is negative (ΔH<0) for the exothermic reactions.
Example:
CH4 + 2O2 = C02 + 2H20 (ΔH = -890kJ)

Endothermic reactions
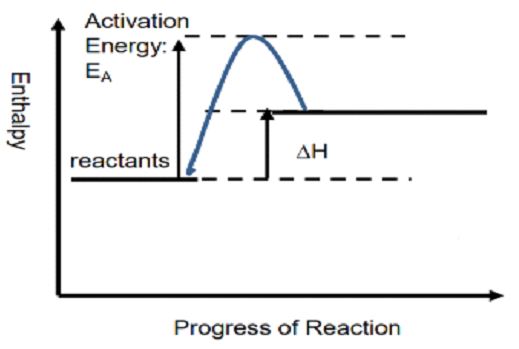
The chemical reaction involving the absorption of heat is called an endothermic reaction. In this case, energy for the reaction is got from the surroundings which mean that energy is provided in the form of heat to carry out a particular reaction. Thus, products have more enthalpy as compared to reactants and ΔH is positive (ΔH>0) for endothermic reactions.
Example:
C02 + 2H20 = CH4 + 2O2 (ΔH = +890kJ)

Hess’s Law
There are many reactions that take place in more than one step for which Hess’s law states that
“Enthalpy change (ΔH) of the overall reaction equals the sum of enthalpy changes of the individual steps” and is represented by the following equation:
ΔHoverall = ΔH1 + ΔH2 + ΔH3 + … + ΔHn
Where “n” represents the total number of steps of that particular reaction.
Calorimetry
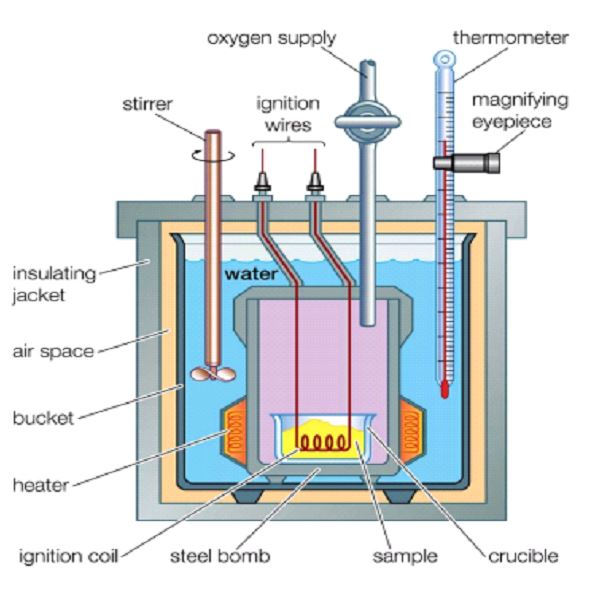
The heat absorbed or released by a chemical reaction is measured by a process known as calorimetry. The device used for this purpose is called a calorimeter.
A bomb calorimeter comprises a container, which is completely closed with an attached thermometer, which measures the heat of the system before and after the completion of a chemical reaction. Thus, when an exothermic reaction takes place the heat released increases the temperature of the surrounding solution (water) while the endothermic reaction absorbs the heat from the surrounding thereby reducing the temperature of the solution. The change in temperature, mass, and the specific heat of the solution is used to measure the overall heat absorbed or released during the reaction.
The measurement is done in calories, where 1 calorie equals the amount of energy that is required to raise the temperature of 1gram of water by 1˚C. As the standard unit of energy is joule (J), 1 cal = 4.18J.

Check out other cool chemistry concepts with other chemistry kits below!