Any kind of system is said to be in equilibrium if it is balanced, for example, two people on the two sides of a seesaw will balance it and it is said to be in equilibrium. Similarly, chemical reactions can also achieve the state of equilibrium, which is called chemical equilibrium.

What is Chemical Equilibrium?
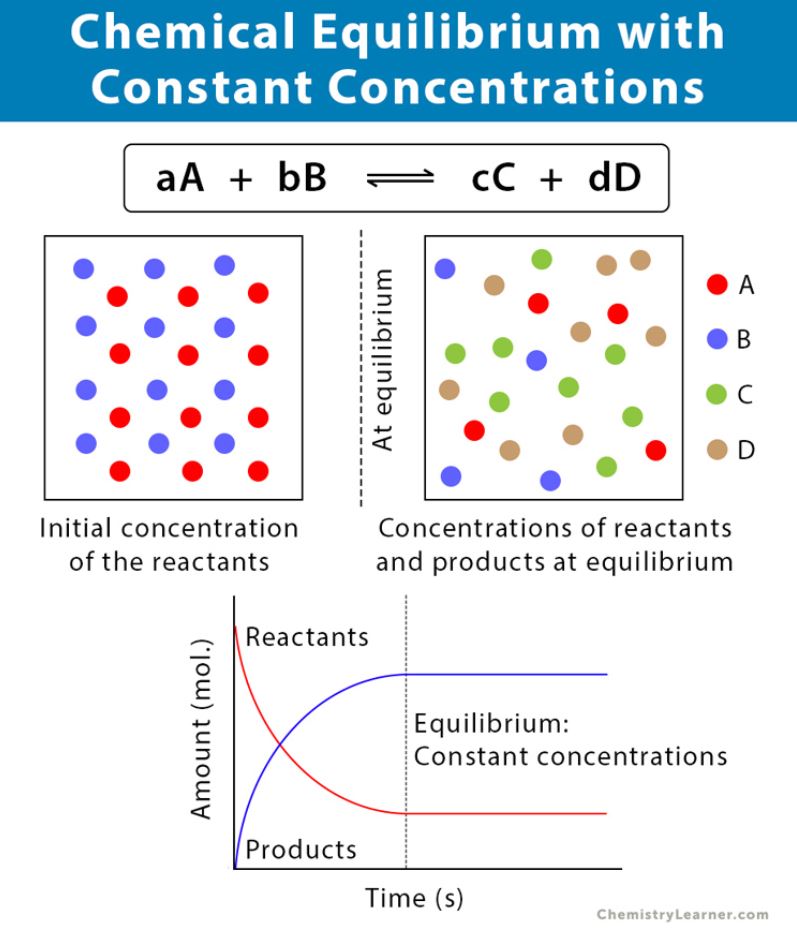
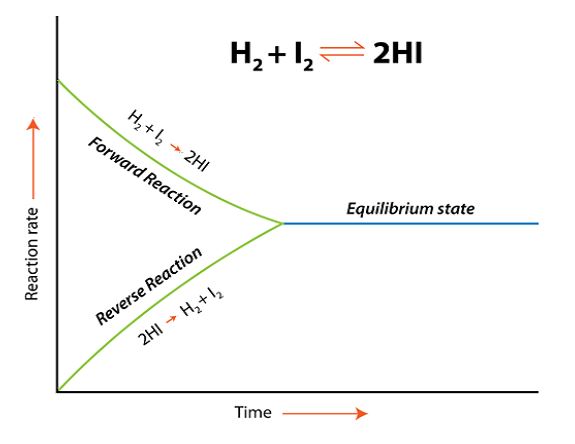
Chemical equilibrium can be defined as that stage of a chemical reaction where the rate of forward reaction becomes equal to the rate of backward (reverse) reaction. At this point, there is no net change in the concentration of reactants and products as the reaction achieves a state of balance. The reaction does not stop after the equilibrium has been achieved but it continues to occur at a constant rate therefore it is also known as dynamic equilibrium.
Properties of the System at Equilibrium
⦁ There should be a closed system allowing no substance to enter or leave the system.
⦁ Rates of forward and reverse reaction should be equal.
⦁ The concentration of reactants and products must not have to be equal but should remain constant.
⦁ Being a dynamic process the forward and reverse reactions continue to take place.
Example:
H2(g) + I2(g) ⇌ 2HI(g)
Initially, only forward reaction takes place and HI is formed:
H2(g) + I2(g) 2HI(g)
Once HI is formed, it is decomposed back to H2 and I2 until equilibrium is achieved:
2HI(g) H2(g) + I2(g

Types of Chemical Equilibrium
Depending on the phase of reactants or products, chemical equilibrium can be divided into the following two types:
⦁ Homogenous Chemical Equilibrium: It is the type of chemical equilibrium in which the reactants and products are present in the same phase. For example:
N2(g) +O2(g) ⇌ 2NO(g)
⦁ Heterogeneous Chemical Equilibrium: The reactants and products in this type of chemical equilibrium occur in different phases. For example:
CaCO3(s) ⇌ CaO(s) + CO2(g)
Equilibrium Constant (Kc)
The equilibrium constant (Kc) is equal to the ratio of the product of concentrations of products to the product of concentrations of reactants raised to the power of their coefficients at a specific temperature and pressure. For a general equation, the equilibrium constant can be written as follows:
aA + bB ⇌ cC +dD
The concentration of reactants and products is measured in mol/L. For gases equilibrium constant is denoted as Kp in terms of their partial pressures. The Kc remains constant unless the temperature of the reaction is not disturbed. Following inferences can be made with the value of Kc:
⦁ Kc > 1 means there are more products present at equilibrium
⦁ Kc < 1 means there are more reactants present at equilibrium
⦁ Kc = 1 means both reactants and products are present at equal concentration
Factors Affecting Equilibrium
The following factors affect the equilibrium of a system:
⦁ Concentration of reactants and Products
⦁ Temperature of system
⦁ Total pressure or volume
When one of these factors changes, the equilibrium of the system is disturbed, and as a result of which the system undergoes readjustments to attain the equilibrium, which is the basis of Le-Chatelier’s principle.
Check out other cool chemistry concepts with other chemistry kits below!