What is Molecular Structure? The molecular structure also known as molecular geometry, depicts how the atoms are arranged in a molecule to form a three-dimensional structure. Determining the molecular structure of different compounds is very important as it tells us about how the molecule reacts, in which phase of matter it falls, its polarity and magnetic properties, and if it has any biological activity.
Bond Angles and Bond lengths
The atoms in the molecules are bonded together by covalent bonds or shared pairs of electrons. As a result of these directional bonds, atoms assume definite positions in comparison to each other strengthening the bonds. This arrangement and bonding of atoms give molecules a specific and rigid structure in which atoms are spatially distributed. Therefore, before predicting the molecular structure of a compound, we should know how the atoms in that particular molecule or compound are bonded together.
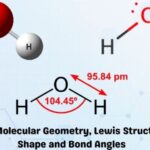
Bond Angle: The angle between two bonds in a molecule shared by a common atom is called the bond angle. It is measured in degrees.
Bond Length: It is the measure of distance between the nuclei of two atoms bonded together in a straight line. It is measured in Angstrom (Å)
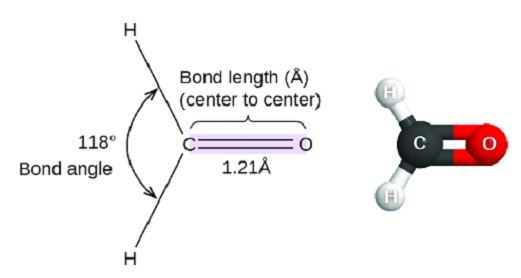

Prediction of Molecular Structure
Lewis-Dot Structure: It determines the two-dimensional structure by illustrating all the valence shell electrons around each atom in a molecule and the bonding between atoms. The lone pairs (not involved in bonding) are indicated in the form of dots as shown below:
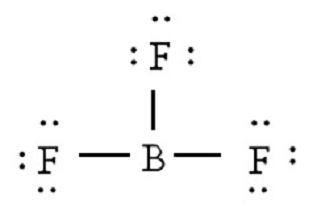
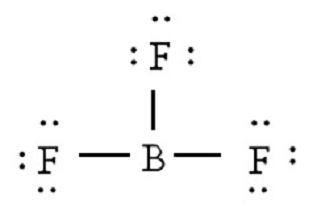
VSEPR Theory
Using information from the Lewis structure, the molecular structure of any chemical compound can be predicted by valence shell electron repulsion theory (VSEPR) by locating the valence electrons around each central atom. According to this theory, the electron pairs, bonded or present as lone pairs repel each other, and to reduce these repulsions they arrange themselves in such a way that increases the distance between them. All the electron pairs (lone and bond pairs) around central atoms are located using VSEPR theory and the structure of the molecule is predicted. The structures predicted are also known as electron-pair geometries, which are similar to the molecular structure unless there is no lone pair of electrons. Molecular structure only shows the placement of atoms and not electrons.
The following 5 molecular geometries are predicted by VSEPR theory:
Linear Geometry: Two regions of electron density around the central atom. For example, the molecular structure of a gaseous molecule BeF2:
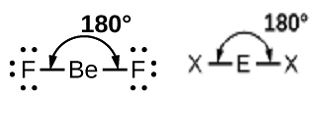
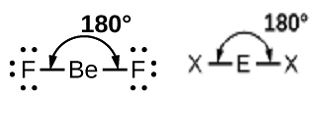
There are two bond pairs and no lone pair of an electron around a beryl atom.
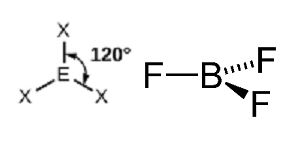
Trigonal Planar: Three regions of electron density around the central atom. For example molecule of BF3.

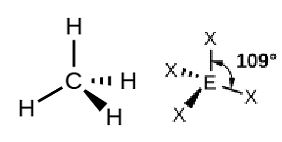
Tetrahedral: Four bonded electron pairs around the central atom. For example, the CH4 molecule.

Trigonal bipyramid: Five bond pairs around the central atom. For example the molecule of PCl5.
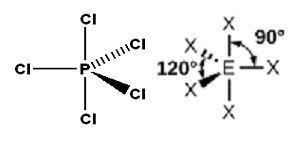

Octahedral: Six bonds around the central atom. For example, depicted by a molecule of SF6.
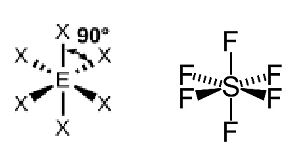
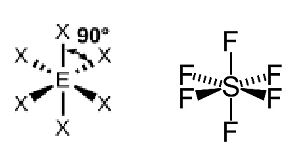
Check out other cool chemistry concepts with other chemistry kits below!