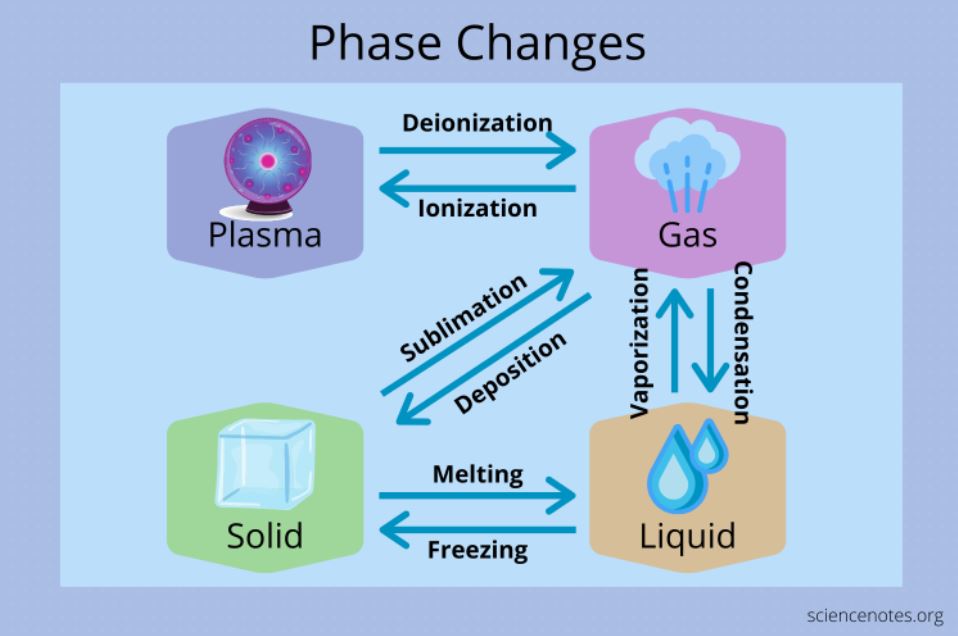
Changes in a matters state (from solid to liquid to gas or plasma) are known as “phase changes” in scientific terms. Changes like these occur when the system is given or taken a large amount of energy, or when the pressure on it is altered. The temperatures and pressures at which these changes take place are determined by the chemical and physical features of the system.
There are three primary states of matter at given temperatures: solid, liquid, and gas (plasma, a fourth state of matter, is energized gas). Intermolecular forces acting on a material’s molecules and atoms dictate the temperature and pressure at which it changes. In a single container, two phases can exist at the same time. The transition from one phase to another is a common occurrence in chemical reactions. This is referred to as a dual-phase condition. For example, during the process of melting ice, includes both solid and liquid water.
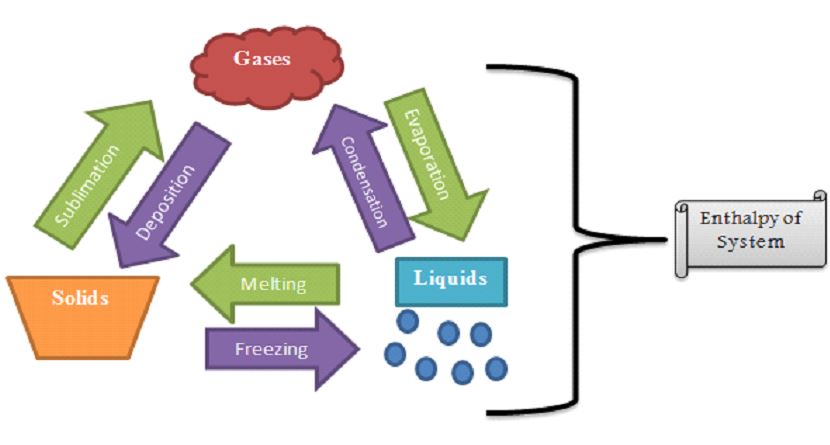
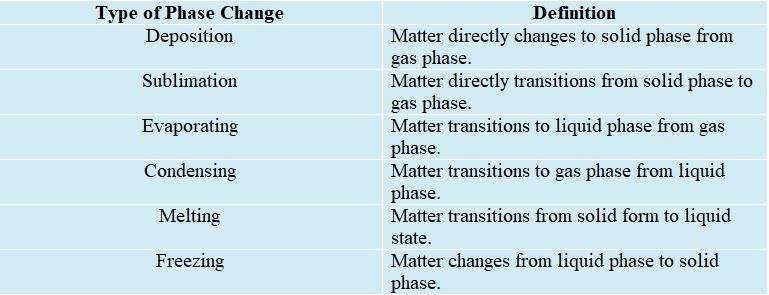
Melting, freezing, evaporating, condensing, sublimating, or depositing are all ways for a substance to transition between these three states. Each of these processes is reversible and proceeds in a separate manner from one phase to the next:

Type of Phase Changes Defined

Examples of Phase Changes
Everyday, you probably encounter different example of phase changes. The process of freezing transforms liquid water into ice cubes. Ice cubes cease to be solid and begin to turn into liquids when they melt. Condensation is the process by which dew forms on grass in the morning. When water boils, either cooking or when you are in the shower, it produces steam. This process is known as “vaporization.” Deposition happens when water vapor undergoes a process that directly leads to the freezing point, like when frost forms on a cold winter morning. When dry ice is heated, it becomes gas nearly immediately. This is an instance of the process known as “sublimation.” By undergoing a metamorphosis, gas can be turned into plasma. To achieve this, it will be necessary to inject a significant amount of energy into the gas in order to eject the electrons from their atoms’ orbits.
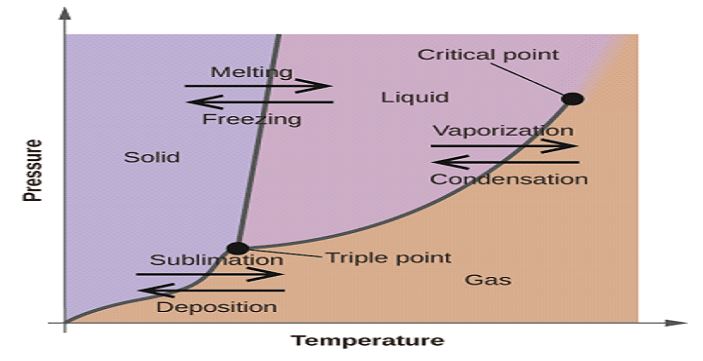
Phase Change Diagram
A phase diagram illustrates how the physical properties of a substance change depending on the temperature and pressure of the environment. The y-axis of a typical phase diagram depicts the pressure, while the x-axis denotes the temperature. A phase shift takes place whenever the lines or curves that make up the phase diagram are intersected with one another.

Check out other cool chemistry concepts with other chemistry kits below!