Hello and welcome to Teach Kids Chemistry! Today, we will be exploring one of the most fascinating elements in the periodic table – Helium. Helium is a colorless, odorless, and tasteless gas that is the second lightest element in the universe. It is also the second most abundant element in the observable universe, after hydrogen. In this overview, we will learn about the properties, uses, and interesting facts about helium. So, let’s dive in and discover the wonders of this amazing element!
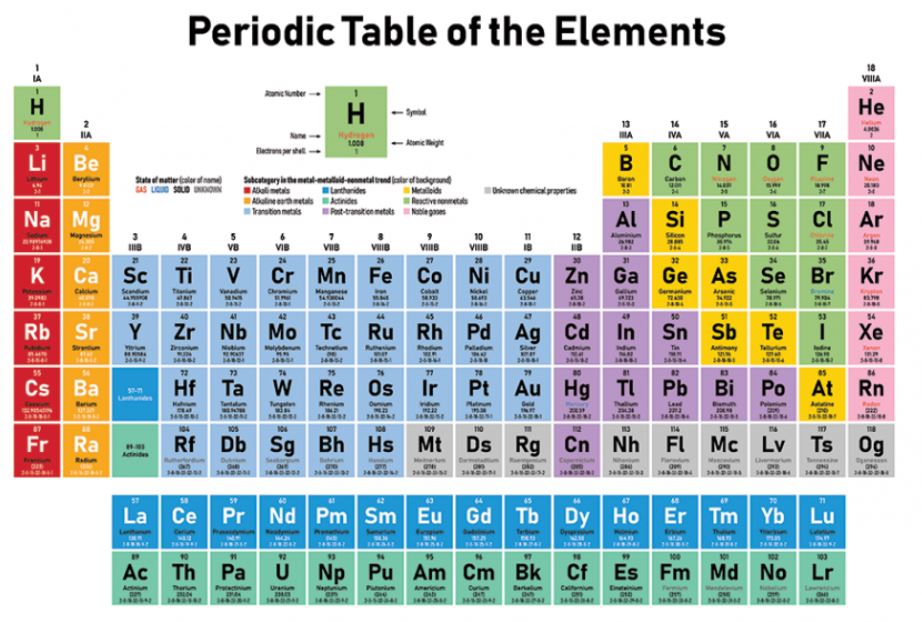
The Periodic Element Helium Overview
Helium is a chemical element with the symbol He and atomic number 2. It has an atomic mass of 4.0026 u. Helium has 2 protons, 2 electrons, and usually 2 neutrons (although it can have 1 or 0 neutrons in some isotopes). It is located in period 1 and group 18 of the periodic table. Helium is a noble gas and is therefore classified as a nonmetal. It has a very low electronegativity and does not readily form compounds with other elements. Helium has a very low specific heat capacity, which means it requires very little energy to change its temperature. Its melting point is -272.2°C and its boiling point is -268.9°C. Helium is a gas at room temperature and pressure, and it is the second lightest element in the universe after hydrogen. Its density is very low, at 0.1785 g/L, which is why it is often used to fill balloons and airships. Helium is also used in cryogenics, as it can be cooled to very low temperatures without solidifying.
Everyday objects that contain the periodic element helium?
There are many everyday objects that contain chemicals or compounds that can be used to teach chemistry concepts. For example, water is a compound made up of two hydrogen atoms and one oxygen atom, and can be used to teach about chemical formulas and the properties of compounds. Salt, which is made up of sodium and chlorine ions, can be used to teach about ionic bonding and the properties of solutions. Baking soda, which is sodium bicarbonate, can be used to teach about chemical reactions and the properties of acids and bases. Other examples include vinegar, which is acetic acid, and aspirin, which is acetylsalicylic acid. By using everyday objects that contain chemicals, students can learn about chemistry concepts in a simple and relatable way.
Differences in the periodic element helium across states of matter
The state of an element can vary greatly depending on its temperature and pressure. At standard temperature and pressure (STP), most elements are either solids or gases. Solids have a fixed shape and volume, while gases have neither. As temperature and pressure increase, some solids can become liquids, which have a fixed volume but take the shape of their container. As temperature and pressure continue to increase, some liquids can become gases, which have neither a fixed shape nor volume. At extremely high temperatures and pressures, some gases can become plasmas, which are highly ionized and conductive. Plasmas are often found in stars and lightning bolts, and have unique properties such as the ability to emit light.
Is the periodic element helium dangerous or radioactive?
No, helium is not dangerous or radioactive. It is a non-toxic, non-flammable, and inert gas that is commonly used in balloons, blimps, and airships. Helium is the second lightest element in the periodic table and is produced by the radioactive decay of heavy elements such as uranium and thorium. It is also found in natural gas deposits and is extracted through a process called liquefaction. Helium has many important industrial and medical applications, including cooling and pressurizing rocket engines, MRI machines, and welding equipment. Overall, helium is a safe and useful element that poses no significant health or environmental risks.
Is the periodic element helium rare and expensive?
Helium is a relatively rare element on Earth, but it is not necessarily expensive. It is the second lightest element and is present in the atmosphere in small amounts. However, it is extracted from natural gas reserves and is widely used in various industries, including medical, scientific, and technological applications. While the cost of helium can vary depending on supply and demand, it is generally not considered an expensive element.
Learn about all the elements with a periodic table!