What is meant by Periodicity? Ever wonder why the elements in the periodic table are in the order they are in? In the context of the periodic table, periodicity denotes the common trends seen in the properties of elements arranged in the increasing atomic number. As we move from top to bottom or left to right in a periodic table, there are certain trends seen in the chemical and physical properties of elements. The periodicity of properties in the periodic table reflects the Periodic law according to which the chemical and physical properties of the elements are the function of their atomic number.
Significance of Periodicity
The modern periodic table is the reflection of periodicity as elements in the groups share similar chemical properties, while those in the periods have similar electron filling in the shells. Due to this, it has become very easy to predict the properties of various elements even if they are newly discovered. Periodicity also helps in determining the possible chemical reactions and bond formation.
Periodic Properties and their Trends
Periodic properties are various physical and chemical properties that are repeated after regular intervals in the periodic table. The key trends or periodic properties are shown by elements in the periodic table are:
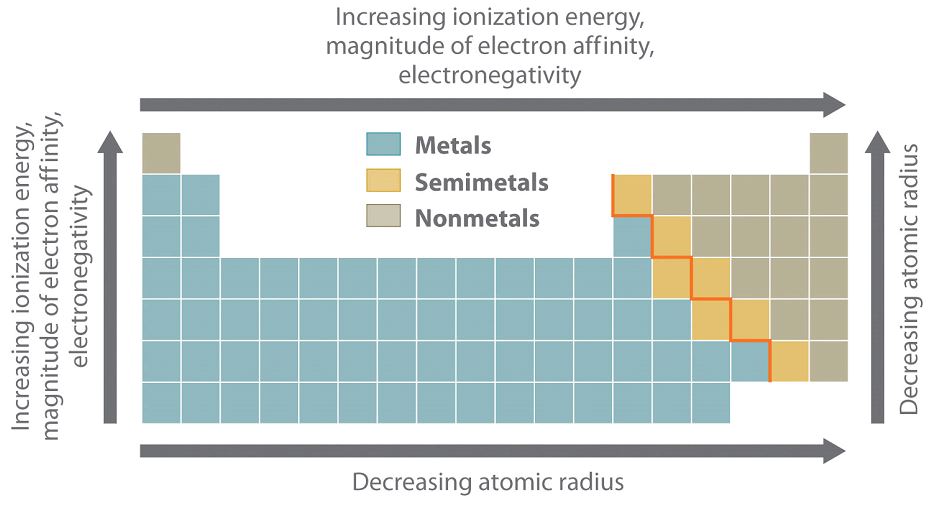
⦁ Electronegativity: Electronegativity is defined as the ability of an atom to form a chemical bond by attracting the shared pair of electrons towards itself. As we move down in a group, electronegativity decreases due to an increased atomic size, which reduces the ability of an atom to attract the shared pair of electrons. On the other hand, electronegativity increases from left to right in a period due to decreased atomic size.
⦁ Ionization Energy: It is the energy required to remove an electron from the outermost shell of an atom when it is in the ground state. For example:
Na Na+ + 1e- I.E = +496Kjmol-1
Ionization energy also decreases as we move down the group due to increased atomic size and increases along the period as the atomic size decreases.
⦁ Electron Affinity: It is the measure of energy that is absorbed or released when an atom accepts an electron in an isolated form resulting in a negatively charged ion. For example:
Cl + 1e- Cl- Electron affinity = -349Kjmol-1
Like electronegativity and ionization energy, electron affinity also decreases as we move from top to bottom and increases across the period. Metals are known to have lower electron affinities as compared to nonmetals. In addition, as noble gases have already filled their outermost shell so their electron affinity approaches zero.
⦁ Atomic Radius: Half of the distance between the nuclei of two atoms that are bonded together or adjacent to each other is called the atomic radius. It is measured in picometers (pm) and increases as we move down the group due to the addition of an electronic shell at each step. It decreases across the period due to increased nuclear charge which pulls the valence shell towards the nucleus, thus decreasing the radius.
Periodicity Summary
Across periods:
⦁ Ionization energy increases
⦁ Electron affinity increases
⦁ Electronegativity increases
⦁ Atomic radius decreases
Down the Group:
⦁ Ionization energy decreases
⦁ Electron affinity decreases
⦁ Electronegativity decreases
⦁ Atomic radius increases

Check out other cool chemistry concepts with other chemistry kits below!

