You might have used the concept of stoichiometry to double a cake recipe, as it is similar to a balanced chemical equation, which represents a specific type of reactants (ingredients) that will combine to produce a particular product (cake). In addition, it also tells us about the number of reactants used to produce a particular amount of products (number of flour cups to make a cake of 1 pound). Here’s a simple example with grilled cheese:
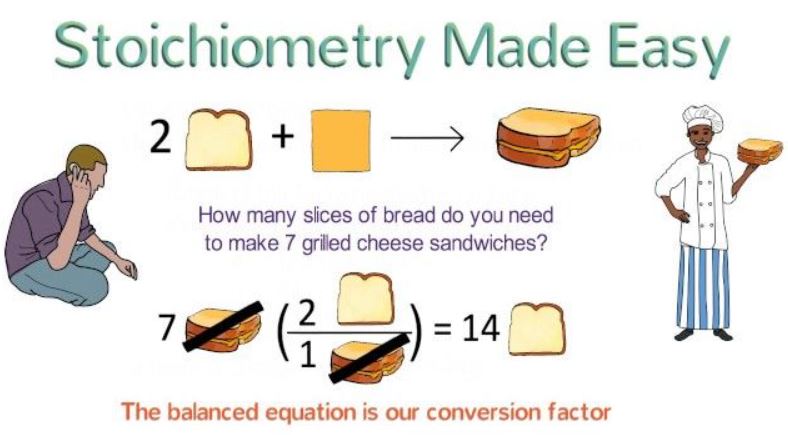

What is Stoichiometry?
Stoichiometry is the branch of chemistry that deals with the numerical relationship between the reactants and the products in a particular chemical equation. The word stoichiometry is derived from two Greek words i.e. stoikhein (meaning element) and metron (meaning measure). So, the literal meaning of stoichiometry is the measure of elements.
Now! Let us learn how stoichiometry can be used to determine the quantitative data about reactants and products during a chemical reaction!
Chemical Reaction and Balanced Stoichiometry
A chemical reaction can move forward and be completed, only if it has balanced stoichiometry. This means that the number of elements formed in the products should be equal to the number of corresponding elements in the reactants. For example, if a chemical reaction begins by using 6 carbon atoms as in the photosynthesis reaction, it should also end with 6 carbon atoms in the products as follows:
6CO2 + 6H2O C6H12O6 + 6O2
This number written in front of molecules is called the stoichiometric coefficient, which can be defined as the number of molecules participating in a reaction. By keenly observing the above chemical equation you can notice that the number of elements in the reactants is equal to the corresponding number of elements in the products.
The concept of Mole Ratios
Mole ratio is an important concept of stoichiometry, which is used to relate the number of moles of a pair of substances in a chemical reaction. As one mole is equal to 6.022×1023 and the mass of one mole of a substance is called molar mass (which equals to molecular formula mass), molar ratios can be used to calculate the mass in grams of reactants and products as follows:
3Fe + 4H2O Fe3O4 + 4H2
Here 3 moles of iron and 4 moles of water are used to form 1 mole of iron oxide and 4 moles of hydrogen, which depicts that 3×56 (168g) of iron and 4×18 (72g) of water react to produce 231 g of iron oxide and 4×2 (8g) of hydrogen gas.
Limiting Reactant and Percentage Yield
In a chemical reaction, one reactant is present in excess while the other is consumed as the reaction stops. The latter is called limiting reactant as it limits the concentration of product formed.
N2 + 3H2 2NH3
In the above reaction, hydrogen is the limiting reactant as one mole of N2 requires 3 moles of H2 to produce 2 moles of NH3.
The yield calculated by stoichiometric analysis (theoretical yield) is not equal to the actual yield therefore, percentage yield can be obtained by the following formula:
Using the above-mentioned stoichiometric concepts, we can solve various problems related to chemical reactions.
Check out other cool chemistry concepts with other chemistry kits below!

